Biggest leap in identified lung health genes paves way for personalised risk score
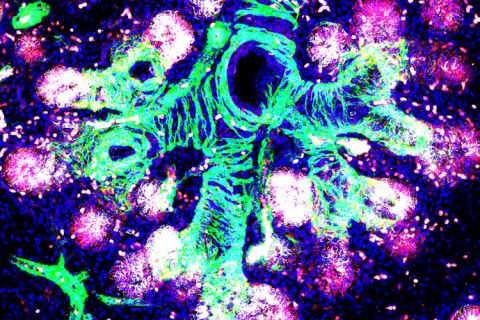
Image shows a 16 weeks post-conception human fetal lung. The branching airway tree is surrounded by bands of developing smooth muscle (green), which also labels the developing vasculature. The epithelial progenitor cells (co expressing SFTPC in white and NOTUM in red) are initiating the process of alveolar epithelial development.
Image supplied by co-author Emma Rawlins (University of Cambridge), image credit Dr Kyungtae Lim.
Over 500 new genes have been linked to lung function for the first time, allowing scientists to better understand lung disease.
The study, led by the University of Leicester and University of Nottingham, is the largest and most diverse study of its kind. The findings pave the way for potential new treatments to tackle conditions such as chronic obstructive pulmonary disease (COPD) and asthma, and highlight existing drugs that could potentially be repurposed at speed.
Published today (13 March) in the journal Nature Genetics, the study analyses genome data from 580,869 participants worldwide to build the most confident picture yet of how our genes affect our lung health.
Lung function tests analyse how well the lungs move air in and out of the body. In conditions such as asthma and COPD this is made more difficult by narrowing of the airways. Chronic respiratory disease, such as COPD, is the third leading cause of death globally.
Establishing which genes are involved in lung function is important as it is these genes that encode the proteins that medicines target to prevent or treat disease. Using a new approach that brings together big data relating to genetic variation, lung health and the influences of genetic variation on gene function, this study has been able to identify 559 new genes implicated in lung function with greater confidence than ever before.
It is a huge boost to scientists as they seek to understand which medicines may help improve lung health, and also which medicines might make lung health worse.
Principal Investigator on the study, Professor Martin Tobin from the University of Leicester Department of Population Health Sciences, said: “This is a big leap in terms of the size and the ethnic diversity of the populations that we've been able to study before and it's a huge step in the number of associated genetic variants that we've discovered.
“Our genetic research findings can be used to generate individual risk scores that could personalise medicine. At this stage the risk scores we developed form important tools for further research, but in the future these could help to select which drugs might be most effective for individual patients, and which drugs should be avoided.”
The study combined genomic information from multiple research studies worldwide as part of the SpiroMeta consortium and the CHARGE consortium. This provided the researchers with the most ethnically diverse population for this type of study to make their analysis. The study was funded by Wellcome and supported by the National Institute of Health and Care Research (NIHR) Biomedical Research Centres in Leicester and Nottingham.
The University of Nottingham lead Professor Ian Hall commented: “Inclusion of people from diverse backgrounds in genetics research is important to make sure that all groups of people benefit from the advances in prevention and treatment that such research can bring. At present though, the majority of people in genetic studies are from white backgrounds. In the future, we urgently need more studies in different ethnic groups to provide the necessary sample sizes to really take the field forward.”
The discoveries come as the researchers begin an £8.8 million project funded by Wellcome aiming to identify drug targets and accelerate the process of developing new treatments for lung conditions by combining expertise in data science, genomics and cutting-edge laboratory techniques in a ‘one-stop shop’.
Professor Tobin adds: “Bringing teams from the Universities of Leicester, Nottingham and Cambridge with expertise spanning genomics, data science and cell biology into a single project means that we can speed up the steps leading to new treatments. This Wellcome funding also means that we will be able to improve the diversity of genomic studies in the UK, such as the EXCEED study, and boost genomic studies of lung disease in under-served populations worldwide.”